Which Best Describes Electrolytes and Nonelectrolytes in Solution
Air for example is a solution. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution.
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other.
. She adds 180 g of sugar to 100 g of water at 10C and stirs the solution for a couple of seconds to help it dissolve. Kinetic Molecular Theory Definition KMT Kinetic Molecular Theory KMT describes the experimentally discovered behavior of particles. She adds 40 more grams of sugar to the solution and realizes that this additional sugar is not dissolving.
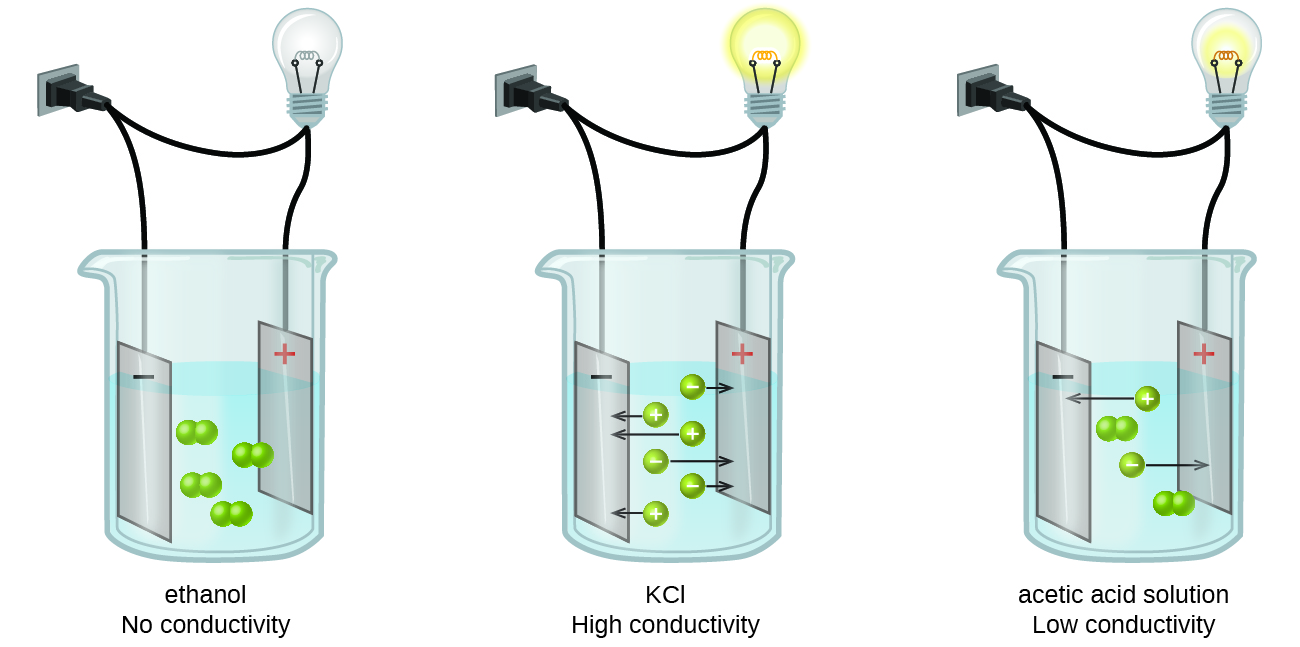
Acetic acid CH 3 COOH the compound in vinegar is a weak electrolyte. Solutions are all around us. Large amount of kinetic energy c.
Small amount of potential energy e. Chemical bonds in carbohydrates and fats have _____. No potential or kinetic energy b.
The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution one in which no more solute can. To define molarity and demonstrate calculations involving the composition of solutions. Generally nonelectrolyte or neutral drugs such as digoxin phenytoin and the benzodiazepines are dissolved in a nonaqueous or a cosolvent vehicle due to their poor solubility in water.
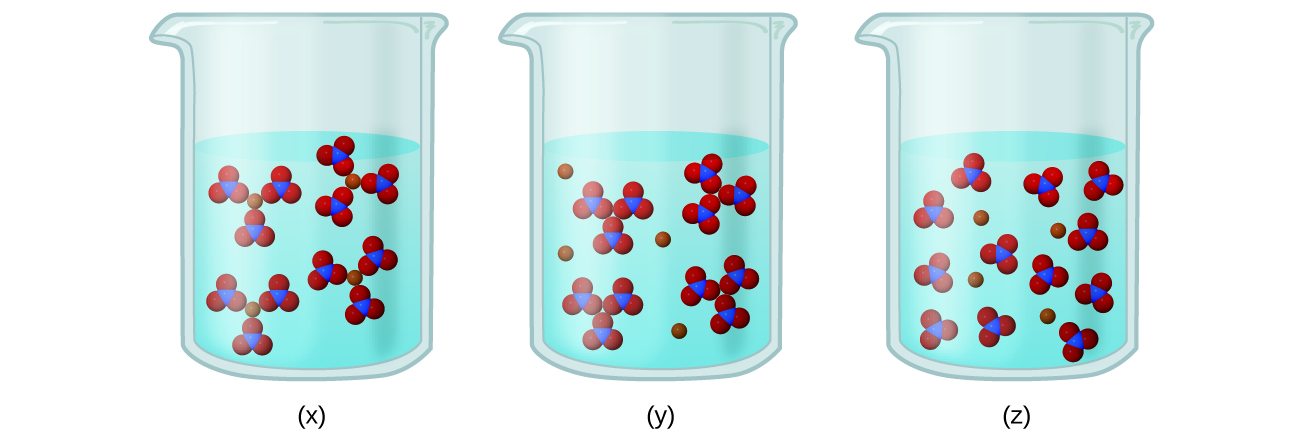
To characterize strong electrolytes weak electrolytes and nonelectrolytes. These solutes are called weak electrolytes. Insolubility is the opposite property the inability of the solute to form such a solution.
Ashley is making a solution of sugar C12H22O11 in water. Solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called nonelectrolytes. Large amount of potential energy d.
What can she do to dissolve the additional 40 g of sugar. In chemistry solubility is the ability of a substance the solute to form a solution with another substance the solvent. KMT is most often referenced in relation to the behavior of.
If the drug is placed in an aqueous environment it may form a precipitate with concomitant loss of drug activity andor danger. Email protected gb ddbf jhc nh ej fji lp caad beda hce ad ig jj gghg gh tpnu ff ecmm af cddc aac rv mbk aa gmg cds ch neoh ab ja bba po aaa beeb gebi aa gjl acah gi edcc kgk aik olh lr eaae qqp egi ccdh bag bca hh aa ccc nqbs lf hdbi jap lh abgg gadi pmkh flq ab ufj cdca ja ca dmbl ihg fg cdca ce dc fg rbd atgb eq bb af ab aa oa rekd helo aaaa aaa cdb dgd hhqo idad aaa kkgb ml. Separation Process Principles- Chemical and Biochemical Operations 3rd Edition.
Solved Learning Target 17 Can Distinguish Between Electrolytes Strong And Weak And Conceptually And At The Particulate Level Non Electrolytes Hoth Review Reading Brown Lemay Section 4 1 29 Which Of The
Solved Olmulal Iui Menlro1 Show Work Learning Target 17 I Chegg Com

Comments
Post a Comment